VAPE: What’s Legal, What’s Not
Key facts laid out in visual summary of what retailers can and cannot sell
MELBOURNE, Florida – Following up on its recent webcast on the state of vape in 2023, the leadership at Bidi Vapor, LLC, developed a companion infographic summarizing compliance and legal issues surrounding the category. The illustration is available in multiple formats to help the industry understand complexities of bringing electronic nicotine delivery systems or ENDS to market. Click here for downloadable options.
For more information about Bidi Vapor, visit https://bidivapor.com. To view the webcast that is the source for this infographic, click the button to your right.

VAPE:
WHAT'S LEGAL,
WHAT'S NOT
As of the spring of 2023, noncompliant and illegal electronic nicotine delivery systems or “ENDS” devices continue to flood the U.S. market, especially regarding flavors. Confused?
Here’s the law in a snapshot:

3 Zones of Compliance

FDA: PMTA
The FDA’s regulatory process over ENDS.

Synthetic Nicotine
All ENDS with synthetic nicotine are banned1.

PACT Act
Requires proper registration for interstate commerce.
TOBACCO ROAD
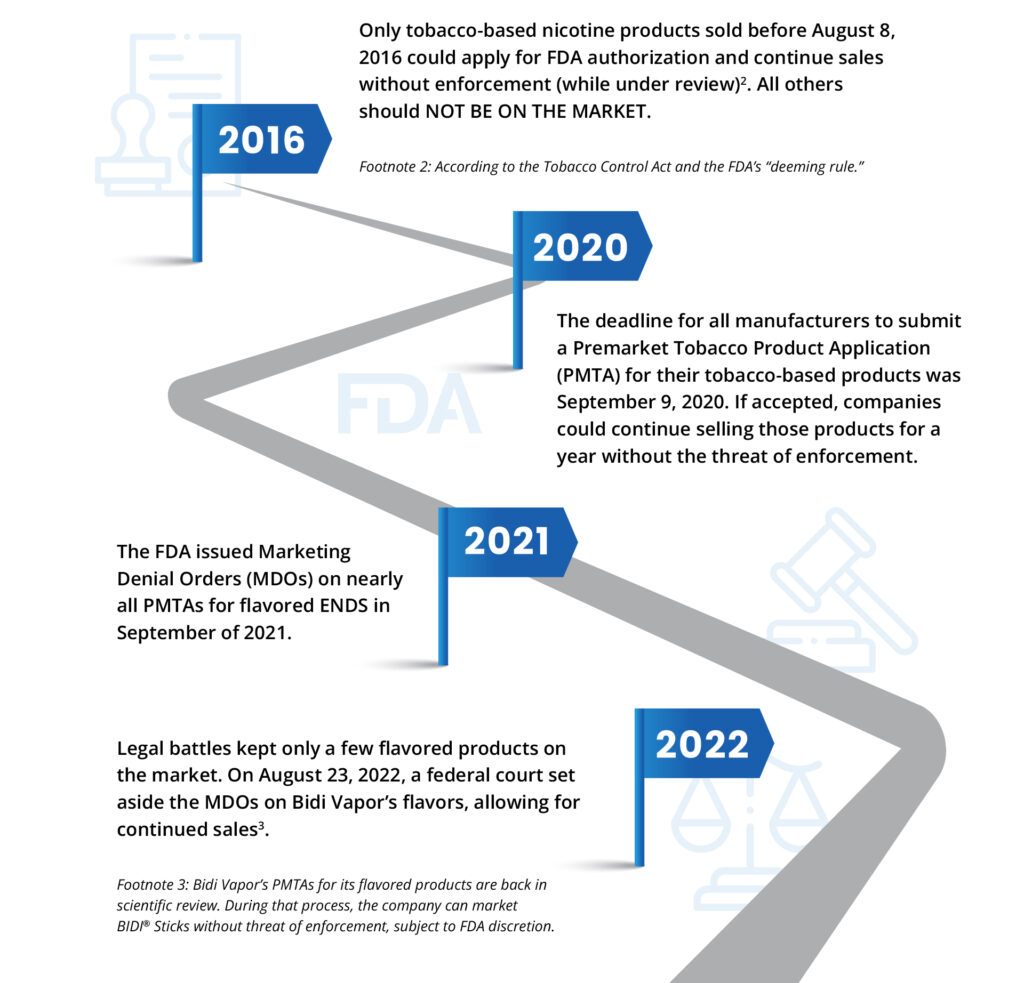

Only tobacco-based nicotine products sold before August 8, 2016 could apply for FDA authorization and continue sales without enforcement (while under review)2. All others should NOT BE ON THE MARKET.
Footnote 2: According to the Tobacco Control Act and the FDA’s “deeming rule.”



Legal battles kept only a few flavored products on the market. On August 23, 2022, a federal court set aside the MDOs on Bidi Vapor’s flavors, allowing for continued sales.
Footnote 3: Bidi Vapor’s PMTAs for its flavored products are back in scientific review. During
that process, the company can market BIDI® Sticks without threat of enforcement, subject to
FDA discretion.
The Story of Synthetic Nicotine



Congress gave FDA authority over all products containing nicotine, regardless of the source.
FDA created a new deadline for PMTAs covering ENDS using synthetic nicotine. Those that complied received a marketing grace period of 60 days.



Grace period ended. After this date, all products containing synthetic nicotine had to COME OFF THE MARKET.
“With that stroke of a pen, synthetic nicotine products became regulated tobacco products4 …”
- Azim Chowdhury, Keller and Heckman, Washington, D.C.

What is the PACT Act
The Preventing All Cigarette Trafficking Act (PACT Act) was created by Congress in 2010 and it authorizes the Bureau of Alcohol Tobacco, Firearms and Explosives (ATF) to prevent criminal organizations from profiting from the illegal sales of tobacco products and imposes penalties for avoiding sales tax payments. On March 27, 2021, Congress included ENDS.
Four major requirements of the PACT Act:



On March 27, 2021, Congress amended the PACT Act to include new regulations regarding the delivery and sales of electronic nicotine delivery systems (ENDS), which include flavored and non-flavored e-cigarettes and vapes (whether or not they contain nicotine), in addition to traditional cigarettes and smokeless tobacco products. Individuals or businesses that sell, transfer or ship for profit any ENDS in interstate commerce, must now register with ATF in accordance with 15 U.S.C. §§ 375 and 376. They must also register with any states that they ship vapes into, as well as report their monthly sales, pay excise taxes and be properly licensed, where applicable. As part of the enforcement mission, ATF regularly collaborates with the U.S. Postal Service (USPS) and Food and Drug Administration's Tobacco 21 enforcement teams to prevent sales and shipments of vapes and e-cigarettes to minors.
Footnote 4: Subject to requirements under the Tobacco Control Act, including premarket review.
Source: Bidi Vapor Webcast: What’s Legal What’s Not? February 7, 2023, Convenience Store Decisions; Featuring Niraj Patel, founder and president of Bidi Vapor, LLC; Russell Quick, president of Kaival Marketing Services (KMS); Azim Chowdhury, partner, Keller and Heckman LLP; and Angel Abecede, press manager, KMS. Link:
https://youtu.be/sXM4BnBZ1RA
How Illegal Products Come to Market
- To avoid fees and taxes, device components enter into the U.S. under false labeling. Assembly happens here.
- Companies receiving warning letters shut down and reopen under a different name.
- Dishonest wholesalers operate separate warehouses off the books.
What are the Penalties?
- Warning letter from the FDA.
- Seizure of product.
- Court-ordered injunctions to stop selling product.
- Criminal prosecution, ranging from misdemeanors to felonies.
- Department of Justice has sued companies that market products without submitting PMTAs.
Where Does Bidi Vapor Stand?
- Its flavored products are legal to sell, subject to FDA enforcement discretion.
- Prioritizes compliance with all federal, state and local regulations and taxes.
- Has a favorable court verdict, setting aside its MDO.
- Focuses on eliminating youth appeal and access.
- Pioneering sustainability and recycling of its products.
- BIDI® Sticks are subject to a comprehensive, pending PMTA with FDA.
- Focus is adult smokers who are unable or willing to quit tobacco or nicotine use entirely.
- Bidi Vapor is continuing to develop science and is publishing studies for peer review.